electron configuration br|Electronic configuration of bromine ? : Pilipinas Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the . Tingnan ang higit pa Classic Pinoy movies are classics for a reason. Although there are newer films featuring new stars, modern graphics, or the latest soundtracks, these Filipino films will always have a special place in our hearts! The characters have spoken to us on another level, and that made us remember their iconic lines forever.
PH0 · How can I figure out the electron configuration of Br
PH1 · Electronic configuration of bromine ?
PH2 · Electron configuration for Bromine (element 35). Orbital diagram
PH3 · Electron Configuration Chart of All Elements (Full Chart)
PH4 · Complete Electron Configuration for Bromine (Br, Br
PH5 · Bromine electron configuration
PH6 · Bromine Electron Configuration :7 Easy Steps on How to Write
PH7 · Bromine Electron Configuration (Br) with Orbital Diagram
PH8 · Br
PH9 · 7.4: Electron Configurations of Ions
Watch Sophieraiin videos and photos brought you by Thotslife.com ☆ Find the best collection of premium Onlyfans leaked, Patreon, Snapchat, Twitch, Nude Youtuber on Thotslife.com .
electron configuration br*******The electron configuration of bromine shows that the last shell of bromine has seven electrons. Therefore, the valence electrons of bromineare seven. The elements that have 5, 6, or 7 electrons in the last shell receive the electrons in the last shell during bond formation. The elements that . Tingnan ang higit paElectronic configuration of bromine ? The total number of electrons in bromine is thirty-five. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in bromine in . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable region of electron rotation around the . Tingnan ang higit pa
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons . Tingnan ang higit pa
Atoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground state electron . Tingnan ang higit pa In this video we will write the electron configuration for Br-, the Bromide ion. We’ll also look at why Bromine forms a 1- ion and how the electron configura. Mar 23, 2023 The electronic configuration for $\ce{Br-}$ is: $$\mathrm{1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^6}$$ Because it have one more electron than bromine, which ends its . Bromine Orbital Diagram. In this article today we are going to tell you about the electron configuration of Bromine, its orbital diagram, and valence electron. Also, we will provide the pictures of the same. .
Orbital diagram. Bromine electron configuration. ← Electronic configurations of elements. Br (Bromine) is an element with position number 35 in the periodic table. Located in the . Br- Electron Configuration (Bromide Ion) Watch on. Methods. Aufbau principle. First, find electrons of bromine atom. Periodic table | Image: Learnool. The atomic number of bromine represents the .
Determine the electron configuration of ions. Justify the observed charge of ions to their electronic configuration. Define paramagnetism and diamagnetism. Justify the . Learn how to write the electron configuration of bromine using the aufbau principle and a chart of subshells. See two answers with explanations and examples on . ground state Bromine orbital diagram. The electrons present in the ground state of Br will consist of 2 electrons in the first shell (K shell), 8 electrons in the second .
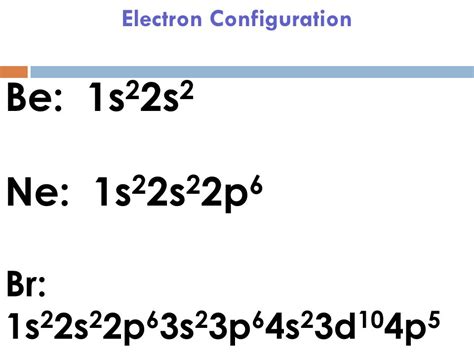
electron configuration brBromine(Br): Bromine is a p-block element having an atomic number 35. Bromine belongs to group 17 which is known as halogens. Electronic configuration of Group 17: The elements of group 17 have seven electrons in their outermost shell. So, the valence electronic configuration of group 17 is n s 2 n p 5. Electronic configuration of Bromine:
The first three quantum numbers of an electron are n=1, l=0, m l =0. Only two electrons can correspond to these, which would be either m s = -1/2 or m s = +1/2. As we already know from our studies of quantum numbers .
The electron configuration of Bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. This can be shortened to #[Ar] 4s^2 3d^10 4p^5#. Explanation: Use a chart such as the one below to fill the subshells in order of the diagonal lines. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.. But wait .Assigning Electron Configuration . We write electronic configurations by following the aufbau principle (from German, meaning “building up”). First we determine the number of electrons in the atom; then we add electrons one at a time to the lowest-energy orbital available without violating the Pauli Exclusion Principle .That is, recognizing that each . Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure.The chemical symbol for Bromine is Br. Electron Configuration and Oxidation States of Bromine. Electron configuration of Bromine is [Ar] 3d10 4s2 4p5. Possible oxidation states are +1,3,5/-1. Electron .How many protons, neutrons, and electrons are in atoms of these isotopes? Write the complete electron configuration for each isotope. Answer. Co has 27 protons, 27 electrons, and 33 neutrons: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 7. I has 53 protons, 53 electrons, and 78 neutrons: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 5.Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right. . CH 3 Br (boiling point 3.5 o C), this has been widely employed to kill pests in the soil, in storage facilities .Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table.. The electron configuration for the first 10 elements. H #1s^1# He #1s^2# Li #1s^2 2s^1# Be #1s^2 2s^2# B #1s^2 2s^2 2p^1# C #1s^2 2s^2 2p^2# N #1s^2 2s^2 2p^3# O #1s^2 2s^2 .

When writing an electron configuration, you have to write serially. Ruthenium ion(Ru 3+) electron configuration. The ground state electron configuration of ruthenium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 7 5s 1. This electron configuration shows that the last shell of ruthenium has an electron and the d-orbital .
The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer .Reduced electronic configuration Br: [Ar] 3d 10 4s 2 4p 5. Below is the electronic diagram of the Bromine atom Distribution of electrons over energy levels in the Br atom 1-st level (K): 2 2-st level (L): 8 3-st level (M): 18 4-st .Write the electron configuration for Br−. There are 2 steps to solve this one. 100 % .
What is the electron configuration for bromine? Chemistry Electron Configuration Electron Configuration. 1 Answer Zach Dec 24, 2015 [Ar] #4s^(2)3d^(10)4p^5# Explanation: All you need to do is work your way across the periodic table filling the orbitals as you go. The full .electron configuration br Electronic configuration of bromine ? Basic Steps. Electron configurations list every subshell for an atom or ion and how many electrons are in each subshell. Subshells are described by writing the principal quantum number n followed by the symbol for the angular momentum quantum number l (s, p, d, or f). The the total number of electrons in each subshell is written as a . An atom's electron configuration describes the way its electrons fill sublevels when the atom is in its ground state. Atoms seek the most stable electron configuration, so sublevels are half-filled or fully-filled .
Bromine (Br) element properties, information, facts, uses and Periodic Table trends. Complete information about the Bromine element - Atomic Number 35, atomic mass [79.904], melting point, How to Locate on Periodic Table, History, Abundance, Physical Properties, Thermal Properties, Crystal Structure, Atomic & Orbital Properties, electron . Well, is it not isoelectronic with krypton..? For atomic Br, we have Z=35, and thus for Br^- we gots 36 electrons to distribute in the usual way... 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)4s^(2)3d^(10)4p^6. Chemistry . . What is the electron configuration of a bromide ion? Chemistry Electron Configuration Electron .
Engineered for convenience. Neverinstall's architecture, built on first principles, offers fast, modern & secure access to virtual desktops that are centrally managed, deployed in multi-cloud & on-premise environments & designed with user simplicity in mind.
electron configuration br|Electronic configuration of bromine ?